This is a issue of definition. TAMC by definition contains yeast and molds. Hence the media should be checked Using these micro-organisms.
That may help you put together for your subsequent audit, we combed via The us Pharmacopeia (USP) chapters associated with GPT. Our overview under consists of the USP chapters an auditor may well reference once they pay a visit to your facility in addition to vital points and essential takeaways for the laboratory.
At our facility, we do not perform pour plates on MacConkey agar. If you think the microorganism is the cause of no growth, please email techsupport@microbiologics.com using this type of concern and we are going to be delighted to investigate this further more.
That is confirmed by identification tests. The solution complies with the test if colonies of the kinds explained are usually not present or Should the confirmatory identification tests are damaging.
Antimicrobial preservatives really should not be applied as an alternative once and for all production practices or entirely to reduce the viable microbial population of the nonsterile solution or control the presterilization bioburden of multidose formulations for the duration of producing.
Even though not a pharmacopeial prerequisite, Microbiologics recommends testing in duplicate in a minimum amount and averaging the outcomes to acquire accurate effects.
Its versatility and talent to guidance the growth of a wide array of organisms ensure it is a worthwhile tool in microbiology laboratories.
We really remember we’ve bought the website owner to become grateful to for that. A lot of the explanations you produced, the clear-cut web site navigation, the associations your site enable instill – it’s largely magnificent, and it’s actually aiding our son along with the family members reckon that The problem is thrilling, that is surely really major. Thanks for The full thing!
Selective media has inhibitory Houses, so it really is to be envisioned which the Restoration is going to be a lot growth promotion test definition less when compared to non-selective media. This can help you save time with investigations and root cause Examination.
atau biasa disebut dengan GPT. Pada pengujian GPT ada beberapa issue penting yang perlu diketahui antara lain:
six. Can we should test systematically in parallel a preceding and approved batch to be able to Review With all the new batch?
Dari hasil contoh GPT pada beberapa merek media TSA di atas terdapat perbedaan jumlah mikroba yang tumbuh, read more jadi dapat disimpulkan bahwa uji
For example, in rooms like ISO five and six, the cfu counts allowable are very reduced and have to be managed quite intently. In USP Microbiological Command and Checking of Aseptic Processing Environments, it states that prompt Preliminary contamination Restoration costs for aseptic environments in ISO five and ISO 6 rooms ought to only display contamination on top of things plates
A Licensed reference product is a reference content characterised by a metrologically legitimate procedure for a number of specified Attributes, accompanied by a certificate that states the value of the desired residence, its associated uncertainty of measurement and an announcement of metrological traceability
 Brian Bonsall Then & Now!
Brian Bonsall Then & Now! Romeo Miller Then & Now!
Romeo Miller Then & Now!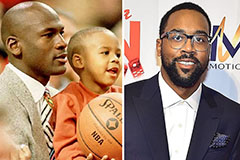 Marcus Jordan Then & Now!
Marcus Jordan Then & Now!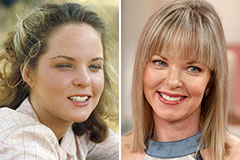 Melissa Sue Anderson Then & Now!
Melissa Sue Anderson Then & Now! Dolly Parton Then & Now!
Dolly Parton Then & Now!